Abstract
Background
Congenital dyserythropoietic anemias (CDA) encompass a heterogeneous group of rare genetic disorders characterized by ineffective erythropoiesis and distinctive multinuclear erythroid precursors in the bone marrow. Clinical findings include chronic anemia, frequently with evidence of hemolysis but with suboptimal reticulocytosis, and iron overload out-of-proportion to transfusion burden. The identification of several genetic defects underlying different subtypes of CDA provided insights into the pathogenesis but many gaps still exist in understanding the related molecular mechanisms and the natural history of the disease.
Objective
To establisha Congenital Dyserythropoietic Anemia Registry (CDAR) in North America with the goal to collect long-term retrospective and prospective phenotypic data and create a bio-repository of de-identified patient specimens as a tool for the investigation of natural history, epidemiology, and biology of CDA.
Design/Method
CDAR was initiated at Cincinnati Children's Hospital Medical Center as a multicenter study in collaboration with the treating physicians (ClinicalTrials.gov Identifier: NCT02964494). Patients of any age diagnosed with CDA and their family members are eligible to enroll. Data including demographic information, medical history, family pedigree and history, diagnostic test results, treatments, and complications are entered in a confidential database with yearly updates. Bone marrow slides are centrally reviewed for confirmation and classification of the diagnosis. The patients may elect to consent to donation of blood, DNA, and bone marrow specimens to the CDAR biorepository and to the generation of induced pluripotent stem cells and/or immortalized B-cells. For participants without a genetic diagnosis, Next-Gen sequencing and deletion/duplication assays for known CDA-associated genes (CDAN1, C15orf41, SEC23B, KIF23,KLF1, GATA1) are used first to identify a mutation. If a causative mutation is not identified, whole exome sequencing may be performed to reveal candidate genes for further research of CDA pathogenesis.
Results
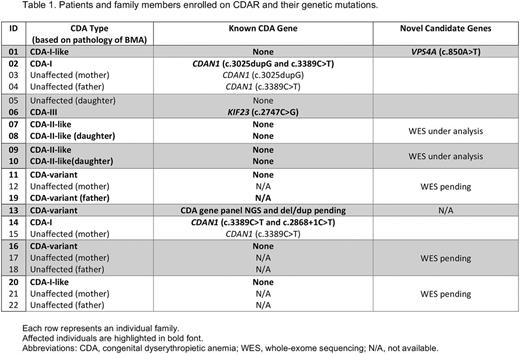
Since CDAR opened in August 2016, 22 participants (13 affected and 9 family members from 10 different families) were enrolled (Table 1). Of the 13 affected individuals, 4 had a pathologic diagnosis of CDA-I, 4 had CDA-II, 1 had CDA-III, and 4 had CDA variants that fit a diagnosis of CDA but did not conform to any of the classical types. Two of the participants with CDA-I found to have pathogenic mutations in CDAN1, one found to have a novel candidate gene variant in VPS4A, currently under validation studies, and one did not have a known mutation and currently undergoing whole exome sequencing. The participant with CDA-III had a mutation in KIF23 . Four individuals, carrying previously a pathologic diagnosis of CDA-II, presented with autosomal dominant inheritance in 2 different families; no mutations in SEC23B or the other known CDA-associated genes have been identified, therefore whole exome sequencing is currently under analysis for novel candidate genes. Finally, the 4 participants with CDA variants are currently undergoing whole exome sequencing.
Conclusion
CDAR will provide a longitudinal database and a biorepository to facilitate natural history studies and molecular pathways research in Congenital Dyserythropoietic Anemias. Such research is necessary to build-up knowledge for these rare diseases in order to guide diagnostic and therapeutic decisions and be a collaborative resource for patients, treating physicians, and investigators.
Rothman: Pfizer: Consultancy; Agios Pharmaceuticals: Honoraria. Chonat: Agios Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal